
Iron
in latin: ferrum

Structure
Properties
Phase solid
| Melting point | 1811 K (1538 °C, 2800 °F) |
| Boiling point | 3134 K (2862 °C, 5182 °F) |
| Density near r.t. | 7.874 g/cm3 |
| when liquid, at m.p. | 6.98 g/cm3 |
| Heat of fusion | 13.81 kJ/mol |
| Heat of vaporization | 340 kJ/mol |
| Molar heat capacity | 25.10 J/(mol·K) |
Applications
-Medical / Biomedical
Surgical instruments and magnetic resonance imaging
-Industrial Engineering
Bridges, steel girders, and machinery
Iron Nanoparticles
Highly reactive, potent, magnetic, and catalytic.
Nickel
in latin: niccolum

Structure

Properties
Phase solid
| Melting point | 1728 K (1455 °C, 2651 °F) |
| Boiling point | 3003 K (2730 °C, 4946 °F) |
| Density near r.t. | 8.908 g/cm3 |
| when liquid, at m.p. | 7.81 g/cm3 |
| Heat of fusion | 17.48 kJ/mol |
| Heat of vaporization | 379 kJ/mol |
| Molar heat capacity |
26.07 J/(mol·K) |
Resists corrosion even at high temperatures.
Applications
-Manufacturing Engineering
Toasters and ovens (withstand heat)
-Industrial Engineering
Batteries
Nickel Nanoparticles
Available as nanofluid and in passivated, ultra high purity, high purity, coated, and dispersed forms. Learn more about nickel here.
Lead
in latin: plumbum
Books on Lead in Kettering's library
Structure

Properties
Phase solid
| Melting point | 600.61 K (327.46 °C, 621.43 °F) |
| Boiling point | 2022 K (1749 °C, 3180 °F) |
| Density near r.t. | 11.34 g/cm3 |
| when liquid, at m.p. | 10.66 g/cm3 |
| Heat of fusion | 4.77 kJ/mol |
| Heat of vaporization | 179.5 kJ/mol |
| Molar heat capacity | 26.650 J/(mol·K) |
Applications
-Industrial Manufacturing
Car batteries (lining) and pipes
-Electrical Engineering
Some solders and in gasoline
Lead Nanoparticles
Used in lead acid, valve regulated lead acid batteries and lithium secondary batteries and nanoscale electronic devices. Learn more about lead here.
Zinc
in latin: zincum

Structure

Properties
Phase solid
Melting point 692.68 K (419.53 °C, 787.15 °F)
Boiling point 1180 K (907 °C, 1665 °F)
Density near r.t. 7.14 g/cm3
when liquid, at m.p. 6.57 g/cm3
Heat of fusion 7.32 kJ/mol
Heat of vaporization 115 kJ/mol
Molar heat capacity 25.470 J/(mol·K)
Applications
-Mechanical Engineering
Zinc galvanizes metals in manufacturing car bodies and suspension bridges.
-Medical
Zinc oxide is used in pharmaceuticals. Zinc sulfide is used in making fluorescent lights and x-ray screens.
Zinc Nanoparticles
Tiny but powerful; highly catalytic.
Copper
in latin: cuprum
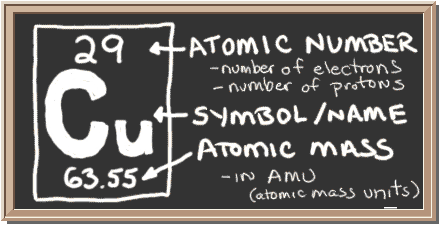
Structure

Properties
Phase solid
Melting point 1357.77 K (1084.62 °C, 1984.32 °F)
Boiling point 2835 K (2562 °C, 4643 °F)
Density near r.t. 8.96 g/cm3
when liquid, at m.p. 8.02 g/cm3
Heat of fusion 13.26 kJ/mol
Heat of vaporization 300.4 kJ/mol
Molar heat capacity 24.440 J/(mol·K)
Good conductor of electricity, does not react with water
Applications
-Industrial Engineering
Water Pipes (low reactivity)
-Electrical Engineering
Wires (good conductor)
The brass used in electrical fittings is 70% copper and 30% zinc
From common household electrical wiring to boat propellers and from photovoltaic cells to saxophones, copper and its alloys are employed in a myriad of end uses. In fact, the metal's use in a wide-range of core industries has resulted in the investment community turning to copper prices as an indicator of overall economic health, spurring the moniker "Dr. Copper."
Learn more about copper and its various applications here.
Copper Nanoparticles
Application research is ongoing to discover the potential dielectric, magnetic, electrical, optical, imaging, catalytic, biomedical and bioscience properties of copper nanoparticles.
Platinum
in latin: platinum

Structure

Properties
Phase solid
Melting point 2041.4 K (1768.3 °C, 3214.9 °F)
Boiling point 4098 K (3825 °C, 6917 °F)
Density near r.t. 21.45 g/cm3
when liquid, at m.p. 19.77 g/cm3
Heat of fusion 22.17 kJ/mol
Heat of vaporization 510 kJ/mol
Molar heat capacity 25.86 J/(mol·K)
Suspended in water
Applications
- Industrial Engineering
Catalytic converters (combustion)
-Industrial Manufacturing Engineering
Textiles, jewelry, and decoration
-Medical / Biomedical
Dental work, medications
Platinum Nanoparticles
Used in magnetic nanopowders, polymer membranes, and cancer therapy. Learn more about platinum here.
Silver
in latin: argentum

Structure

Properties
Phase solid
Melting point 1234.93 K (961.78 °C, 1763.2 °F)
Boiling point 2435 K (2162 °C, 3924 °F)
Density near r.t. 10.49 g/cm3
when liquid, at m.p. 9.320 g/cm3
Heat of fusion 11.28 kJ/mol
Heat of vaporization 254 kJ/mol
Molar heat capacity 25.350 J/(mol·K)
Applications
-Industrial Manufacturing
High capacity silver-zinc and silver-cadmium batteries, solder, brazing alloys, and electrical contacts
-Medical / Biomedical
Dental alloys; silver nitrate is used in oral medicines
-Industrial Manufacturing Engineering
Textiles like the fingertips of gloves used with touchscreen phones and in clothing to prevent some odors
Silver Nanoparticles
Ductile, malleable
Gold
in latin: aurum

Structure

Properties
Phase solid
Melting point 1337.33 K (1064.18 °C, 1947.52 °F)
Boiling point 3243 K (2970 °C, 5378 °F)
Density near r.t. 19.30 g/cm3
when liquid, at m.p. 17.31 g/cm3
Heat of fusion 12.55 kJ/mol
Heat of vaporization 342 kJ/mol
Molar heat capacity 25.418 J/(mol·K)
Very good conductor of electricity, unreactive
Applications
-Electrical Engineering
Connections on circuit boards (conductivity)
-Industrial Manufacturing Engineering
Jewelry (lack of reactivity)
18 carat gold, used in jewelry, is 75% gold and 25% copper and other metals
Gold Nanoparticles
Help to detect breast cancer, toxins, and pathogens in patients. Learn more about the uses of gold here.
Aluminum
in latin: alumen

Structure

Properties
Phase solid
Melting point 933.47 K (660.32 °C, 1220.58 °F)
Boiling point 2743 K (2470 °C, 4478 °F)
Density near r.t. 2.70 g/cm3
when liquid, at m.p. 2.375 g/cm3
Heat of fusion 10.71 kJ/mol
Heat of vaporization 284 kJ/mol
Molar heat capacity 24.20 J/(mol·K)
Low density, does not corrode
Applications
-Industrial Manufacturing
Airplane bodies
Duralumin, used in aircraft manufacture, is 96% aluminum and 4% copper and other metals
Nano Aluminum
Nanoparticle research is an area of strong scientific interest due to the variety of potential applications in optical, biomedical, and electronic fields. In the last 10 years, aluminium/aluminum nanoparticles have been widely researched and used, primarily because of their increased reactivity as compared with conventional micron-sized particle.
Learn about nano aluminum here.